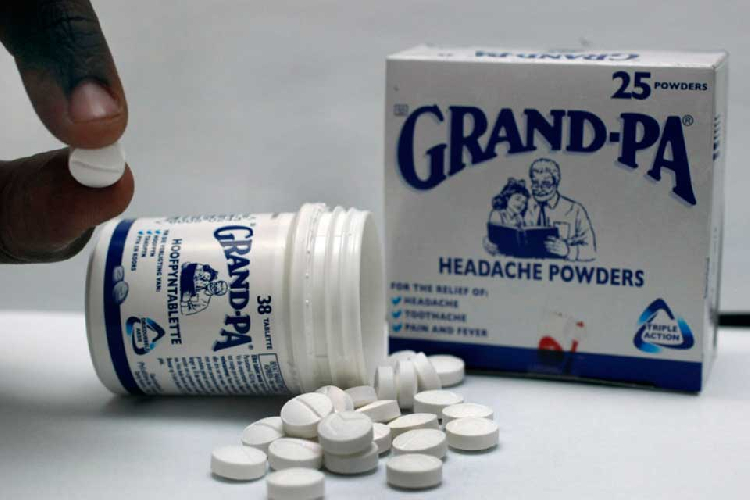
GRANDPA pills and powders have been pulled from Namibian shelves after the medicine failed climatic stability tests.
The Namibia Medicines Regulatory Council announced that all tablets and powders manufactured by GlaxoSmithKline must be recalled.
“The recall is a result of the product failing stability studies at 30 degrees. These products are therefore not fit to be stored under climatic zone IV, in which Namibia is found,” the council’s registrar of medicines, Johannes Gaeseb, said in a statement on Friday.
Gaeseb said these products must not be imported or used in Namibia until further notice.
Stay informed with The Namibian – your source for credible journalism. Get in-depth reporting and opinions for
only N$85 a month. Invest in journalism, invest in democracy –
Subscribe Now!